Spotlight on leishmaniasis in India and the Middle East
Issue: Halting Epidemics
07 February 2017 article

Leishmaniasis is an ancient parasitic disease spread by the bite of an infected female phlebotomine sand fly. This neglected disease, which predominantly affects the very poorest, can manifest as three distinct forms depending on the Leishmania species transmitted: visceral (internal organ damage), mucocutaneous (destruction to mucosal membranes in nose and mouth) and cutaneous (disfiguring skin ulcers). In this article, we briefly review the current status of leishmaniasis in India and the Middle East within the context of disease elimination.
Visceral leishmaniasis (VL)
Apart from malaria, VL is the biggest parasitic disease killer in the world. The visceral form of the disease is the most severe, and in low-income countries the fatality rate can be as high as 100% within two years. Although VL is endemic to over 80 countries worldwide, more than 90% of the world’s cases come from just six countries: India, Bangladesh, Nepal, South Sudan, Brazil and Ethiopia. Of these, nearly 80% of cases come from just one state in India: Bihar, one of the poorest states in a country where 224 million people live below the poverty line.
The name for VL comes from the migration of the parasites into the internal organs of the infected host (viscera = internal organs in anatomy). The Hindi name for VL, kala azar, comes from the words for ‘black’ and ‘fever’ and was named for the dark complexion that many sufferers develop. Other symptoms include prolonged, irregular fever; enlargement of the spleen and liver; and anaemia. When death occurs, it is often due to either haemorrhagic or infectious complications.
Disease transmission control
The elimination of VL is an important public health priority in the Indian subcontinent, and in 2005 the governments of India, Bangladesh and Nepal signed an international agreement which committed them to a partnership using shared methods to achieve elimination by 2015. The strategy so far with patients has been focused on improving early diagnosis and increasing access to treatment. From an insect control perspective, enhancing indoor residual spraying (IRS) of insecticides has helped decrease the population of Phlebotomus argentipes sand flies that transmit the disease. Unfortunately, the target of 2015 was missed. However, the programme to eliminate VL has shown great progress, with a 75% drop in the number of cases since 2005, when the programme was launched.

Early diagnosis
The diagnosis of VL in India has been greatly improved by the adoption of the rk39, a rapid diagnostic test that detects the presence of human antibodies produced in response to Leishmania infection. Prior to introducing the rk39, and in regions where supply of these tests is uncertain, the only method of diagnosis was through biopsy of the spleen followed by microscopic examination of the tissue for the presence of parasites. This procedure requires facilities and expertise that are not frequently present in the rural areas where the majority of cases occur.
Treatment
Historically, treatment for VL in India was long and costly. Treatments using sodium stibogluconate (SSG; Pentostam®, GlaxoSmithKline) and amphotericin B required daily or alternate-day injections for up to 30 days. Additionally, both treatments have the potential to cause side effects such as rigors, fever, and liver, kidney or heart disease, which has been estimated at up to 85% for treatment with SSG and 98% for amphotericin B in India. A newer treatment, miltefosine, can be taken orally and has a lower risk of toxicity, although patients are still required to travel to the local clinic to take the dose under supervision. Better yet is Ambisome™, a preparation of amphotericin B that causes fewer side effects than any other treatment option, but which is prohibitively costly. However, Gilead Sciences has recently announced provision of 380,000 vials of Ambisome™ over the next five years, improving treatment prospects for many patients.
Improving vector control
To reduce the sand fly populations, the Indian Government uses IRS, a vector control technique that sprays insecticide onto the interior walls of houses using pumps carried from house to house by spray operators. India has historically used DDT for its IRS, however, research performed in 2015 showed that the DDT was only active against sand fly populations for one month instead of the hoped-for six. This prompted the Government to change to using a different insecticide – alpha-cypermethrin – and to upgrade the pumps used for the spraying to more modern compression pumps. These advances are expected to achieve elimination in India by 2020.
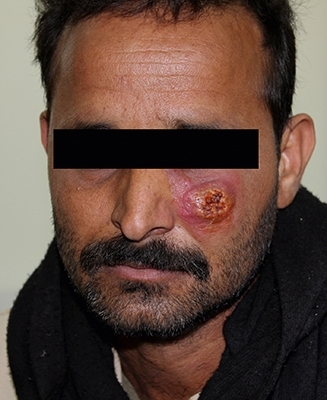
Cutaneous leishmaniasis
Cutaneous leishmaniasis (CL) is the most common form of leishmaniasis as it is prevalent in 98 countries, with more than 1.3 million cases reported annually. With the current political unrest in the Middle East, CL outbreaks are erupting where conflict has disrupted health care systems (including vector control programmes) and forced people to flee into disease endemic areas. Camps filled with displaced persons, military personnel and relief workers are particularly hard hit due to overcrowding, lack of housing infrastructures, and inadequate sanitation, which provide perfect breeding conditions for sand flies and rampant disease transmission.
Diagnosis
Cutaneous lesions can be complicated to diagnose as they can manifest as a range of other dermatological conditions (warts, bacterial abscesses, skin cancer, leprosy, etc.). Typically, patients suffer from painless, raised-edge ulcers that are slow to heal and often infected with secondary microbial infections. These lesions can appear months to years after the infectious sand fly bite and healing often produces unsightly scars. Those living with disfigurement, particularly women and girls, can experience extreme social stigmatisation that can permanently mar life quality with anxiety, depression, and decreased marriage potential. Microscopy and wound examination by specialised dermatologists are the most accessible means of diagnosing CL outside of lab-based assays. In resource-poor settings, where access to such highly trained personnel and microscopes (or even electricity) is limited, the development of new rapid diagnostic tests to not only confirm leishmaniasis, but also discern between the parasite species causing the infection, is needed. This latter point is very important as patient treatment responses vary considerably since Leishmania species can have different drug sensitivities. Serological (blood-based assays) testing can also provide diagnostic information, although discerning an active infection from a latent infection can be complicated.
Treatment
Although CL is often self-clearing, long-lasting lesions (can be years) produce significant scarring and it is possible that it can spread to the more invasive, mucocutaneous form if left untreated. Topical therapies include cryotherapy, thermotherapy (including burning) and surgical excision. As with VL treatment regimes, SSG is administered to patients with CL lesions that do not heal after initial antibiotic/antifungal treatment. Weeks of painful intralesional or systemic injections (sometimes several courses) produce high cure rates, but in resource-restricted settings, such treatments can be logistically difficult.
Media representation
Unfortunately, recent world media spotlights have falsely branded CL as a “flesh-eating disease” or “zombie disease”. Images of disfigured patients with Gentian violet-stained facial lesions, combined with sensationalised journalism, have further heightened xenophobic sentiments and fear in host countries accepting refugees. It is worth emphasising that CL can only be transmitted via sand flies; patients need access to treatment, not a quarantine.
Leishmaniasis prevention
As there are no prophylactic drugs and promising vaccine candidates have yet to manifest into phase III clinical trials, prevention is the most effective way to reduce disease burden. In the poorest regions, this can only be achieved by removing sand fly and animal reservoir populations from human settlements; eliminating sand fly habitats; and implementing disease/entomological surveillance strategies so that insect control and public health interventions can effectively target those areas at highest risk.
Lee Haines & Geraldine Foster
Liverpool School of Tropical Medicine, Pembroke Place, Liverpool, L3 5QA
[email protected]
[email protected]
Further reading
Al-Salem, W. & others (2016). Cutaneous leishmaniasis and conflict in Syria [letter]. Emerg Infect Dis 22(5), 931–933.
Coleman, M. & others (2015). DDT-based indoor residual spraying suboptimal for visceral leishmaniasis elimination in India. Proc Natl Acad Sci U S A 112(28, 8573– 8578.
Gillespie, P. M. & others (2016). Status of vaccine research and development of vaccines for leishmaniasis. Vaccine 34(26), 2992–2995.
Mondragon-Shem, K. & Acosta-Serrano, A. (2016). Cutaneous leishmaniasis: the truth about the ‘flesh-eating disease’ in Syria. Trends Parasitol 32(6), 432–435.
World Health Organization (2016). South-East Asia poised to defeat visceral leishmaniasis (kala-azar). Accessed 2 December 2016.
World Health Organization (2016). WHO and Gilead Sciences extend collaboration against visceral leishmaniasis. Accessed 2 December 2016.


