Polio – what are the prospects for eradication?
Issue: Halting Epidemics
07 February 2017 article
Poliomyelitis can be a devastating disease. More commonly known as polio, it can lead to permanent paralysis in approximately 0.5% of cases. Furthermore, in up to 10% of paralytic polio cases, the disease will be fatal. Outbreaks of polio can therefore be crippling not just to individuals but to entire communities.
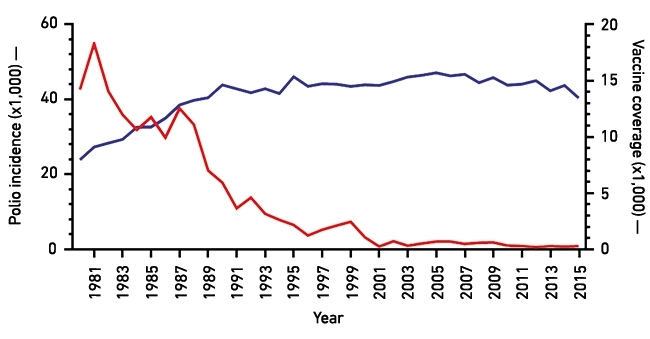
In 1988, the World Health Assembly initiated a programme to eradicate polio. Over the past three decades, the disease has disappeared from most of the world, but it remains endemic in Afghanistan, Pakistan and Nigeria (see Fig.1).
Fig. 1. Effect of immunisation (inactivated polio vaccine, IPC, and oral polio vaccine, OPV) on polio incidence worldwide. Red line, incidence of polio from 1980 to 2015; blue line, vaccine coverage (i.e. use of a combination of OPV and IPV) worldwide.
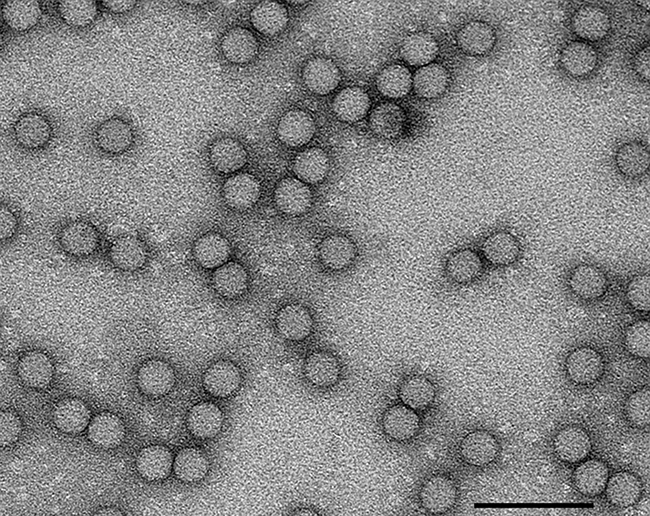
The causative agent is poliovirus (PV), a positive-sense RNA virus in the family Picornaviridae (Fig. 2). It is very easily transmitted and can be spread to vulnerable individuals by direct contact or via contaminated food or water. Worldwide immunisation against polio has been responsible for disease eradication from much of the world, and it is estimated by the World Health Organization (WHO) that since 1988, 5 million people have been saved from lifetime paralysis as a result. The oral polio vaccine (OPV) is composed of live, mutant virus that can infect and reproduce in the human host, but does not cause paralytic disease. It is both cheap to make and easy to administer by mouth in a sugar solution. The use of such oral administration can be undertaken by local volunteers who are not required to be medically trained. This approach allows for the large-scale immunisation that was necessary to eradicate disease in many countries, for example in the Philippines and India. Despite this success and the ongoing efforts of local volunteers, there are challenges that need to be overcome. These are mainly political and geographic. It can be extremely challenging to vaccinate in remote areas, and in some instances, vaccinators have been targeted by radical groups amidst regional conflicts. However, the Global Polio Eradication Initiative, which is led by national governments and supported by five partners (World Health Organization (WHO), Rotary, Centers for Disease Control and Prevention (CDC), United Nations International Children’s Emergency Fund (UNICEF) and the Bill & Melinda Gates Foundation) is committed to continuing immunisation to create a polio-free world.
Fig. 2. Transmission electron micrograph of PV-1 viruses stained with uranyl acetate. Bar, 100 nm.
The OPV is being replaced in much of the world by an inactivated vaccine (IPV) – virus inactivated by treatment with formaldehyde, delivered by injection. The change to such a more expensive vaccine (in terms of both manufacture and delivery) is due to two problems associated with the OPV. The RNA-dependent RNA polymerase that is responsible for making copies of the PV genomic RNA is very error-prone. Therefore, the virus can mutate and/or recombine with circuiting picornaviruses. In rare cases, use of the OPV can therefore cause disease in vaccine recipients or their close contacts (1 in 2.7 million children receiving their first dose of OPV). Secondly, there is a small but important number of immune-deficient or immunocompromised people who are unable to clear the virus after vaccination and therefore persistently shed vaccine-derived virus for long periods of time after immunisation. These individuals would represent a potential source of infection if we were to stop vaccine use. The IPV is not a live virus and therefore does not present either of these risks. However, its manufacture does have considerable biosafety issues as large amounts of virus need to be produced prior to inactivation.
It is clear that we are getting closer to a world without polio, and over the past decade several countries have achieved polio-free status. However, it is also clear that even after wild-type virus is eliminated, we will still need to keep vaccinating for many years in order to avoid outbreaks from vaccine-derived virus.
The WHO is actively involved in funding research into alternative vaccine strategies, which is complicated by there being three distinct PV serotypes. The University of Leeds heads a WHO-funded consortium which aims to develop virus-like particle (VLP) vaccines against polio. VLPs are particles assembled from the proteins that make up the coat, or capsid, of the virus. They contain no viral genomic material, and are therefore both safe to manufacture and to use, as illustrated by the successful human hepatitis B virus and papillomavirus VLP vaccines (Cervarix, Gardasil).
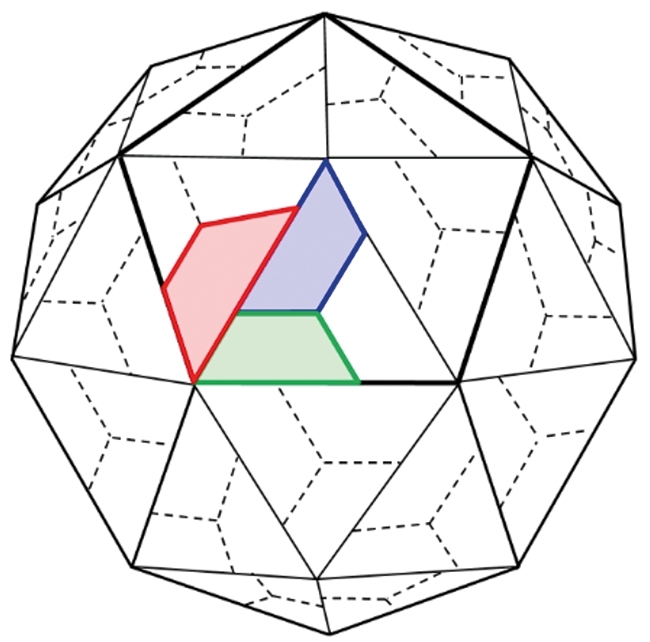
Empty capsids (protein capsids containing no RNA) are made during the normal PV growth cycle. However, these are antigenically unstable, because encapsidation of RNA is required to stabilise the antigenic structure of the particle – the final maturation cleavage step only occurs in the presence of RNA. The capsid proteins VP0, VP1 and VP3 are produced as part of a polyprotein from the P1 region of the genome. VP0 is cleaved into VP2 and VP4 upon RNA encapsidation (Fig. 3). This metastable nature of the PV empty capsid (which still contains VP0) results in a change to a non-native conformation, even at temperatures as low as 10°C – therefore useless as a vaccine. Our approach is to incorporate stabilising mutations into the capsid and express the capsid proteins in a heterologous expression system such as yeast, insect or plant cells. The consortium has used a number of different approaches to arrive at stabilised capsids. These include structure-based design, reversion of temperature–sensitive mutations and using thermal stress to select more stable capsids. We have successfully developed stabilised particles, and some of these are more stable than the formaldehyde-inactivated IPV. The current challenge is to be able to make stabilised VLPs at high yield in our expression systems and translate these into a polio vaccine for the future.
Fig. 3. Cartoon model of the PV capsid. A pentamer is outlined and VP1 (blue), VP2 (green) and VP3 (red) are highlighted.
Oluwapelumi Adeyemi & Nicola Stonehouse
Faculty of Biological Sciences, University of Leeds, LS2 9JT
[email protected]
The WHO-funded VLP vaccine consortium includes:
- University of Leeds: Dave Rowlands, Nicola Stonehouse, Clare Nicol, Oluwapelumi Adeyemi, Lee Sherry
- University of Oxford: Dave Stuart, Liz Fry, Luigi de Colibus, Mohammed Bahar, Claudine Porta
- University of Reading: Ian Jones, Mai Uchida, Sinead Lyons
- The Pirbright Institute: Toby Tuthill, Joe Newman
- John Innes Centre: George Lomonossoff, Johana Marsian
- National Institute for Biological Standards and Control: Andrew Macadam, Phil Minor, Helen Fox, Sarah Knowlson
- Scientific Advisory Board: Jim Hogle, Ellie Ehrenfeld, Jeff Almond
Further reading
Adeyemi, O. & others (2016). Increasing type-1 poliovirus capsid stability by thermal selection. J Virol (in press).
Fox, H & others (2017). Genetically thermo-stabilised, immunogenic poliovirus empty capsids; a strategy for non-replicating vaccines. PLOS Pathogens (in press).
Jacobson, M. F. & Baltimore, D. (1968). Morphogenesis of poliovirus. I. Association of the viral RNA with coat protein. J Mol Biol 33, 369–378.
World Health Organization (2016). WHO vaccine-preventable diseases: monitoring system 2016 global summary.
Wien, M. W., Chow, M. & Hogle, J. M. (1996). Poliovirus: new insights from an old paradigm. Structure 4, 763–767.
Images: Coloured transmission electron micrograph of polio virus particles. Dr Linda Stannard, UTC/Science Photo Library. Fig. 1. Data was collected and collated through six WHO regions (Africa, the Americas, Eastern Mediterranean region, Europe, Southeast Asia and the Western Pacific region). WHO vaccine-preventable diseases: monitoring system 2016 global summary. Fig. 2. Anna Higgins, Sam Stephen & Clare Nicol, University of Leeds. Fig. 3. Virion maturation involves cleavage of VP0 into VP2 and VP4 (which is internal) (Jacobson & Baltimore, 1968). Redrawn by Oluwapelumi Adeyemi, University of Leeds.


